Chemical Changes and Physical Properties
Summary
Students will record observations of the physical properties of a penny and an eggshell. The students will then observe how these two objects react with vinegar. They will then record the physical properties for the two objects again. Students will be able to see the effects of chemical change on physical properties of substances.
Materials
Attachments
- one raw egg
- a clean penny
- paper towel
- 500 ml beaker
- vinegar
- student worksheet (attached)
Background for Teachers
Time Needed: 10-15 minutes at the end of one period (for demo), 20-30 minutes for student observations and answers 2 days later.
The demonstration is safe but can be a little messy. Students should already be familiar with physical properties. The chemical reactions involved are:
- Vinegar (acetic acid) reacts with the Calcium Carbonate in the eggshell to form calcium ions (dissolved in the vinegar) and carbon dioxide, this leaves behind only a membrane.
- Vinegar reacts with the copper on the penny to produce copper acetate which is green in color.
- Another, optional, reaction is a thin chicken bone with vinegar -- essentially the same reaction as the eggshell takes place and the wing-bone becomes rubbery.
Instructional Procedures
- Hook activity -- Give students a small piece of cake, brownie, or chocolate-chip cookie. Ask the students how what they are eating is different than the batter that it came from. (answers will vary). Tell the students they will be learning about how a chemical change (like baking) can change the physical properties of a substance.
- Have the students observe the penny and the egg, have them write down all the physical properties for the two items they can think of.
- Tell the students that the egg and the penny will undergo a chemical change with vinegar.
- Place the egg in a beaker with enough vinegar to cover it completely cover it. Place the penny on a folded paper towel soaked with vinegar.
- Set the two items in a secure place.
- It would be best to do the demonstrations at the end of the week and leave them over the weekend, the eggshell may take up to 3 days to dissolve.
- After the reactions have taken place show the students what has happened and explain the reactions. Have the students observe the penny and the egg again and record all the physical properties they can think of.
- Have the students answer the analysis questions.
Assessment Plan
Attachments
Scoring Rubric:
1. Students fill out observation table completely, correctly……………5
2. Analysis answers show sufficient understanding ..……...5
Possible answers:
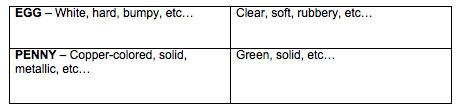
Analysis-- (possible answers)
1. Answers will vary
2. Rock will dissolve, rock will become rubbery, rock will bubble, etc…
3. The reaction also requires oxygen.
4. Students may recall reactions such as something burning, metal rusting, vinegar and baking soda, leaves changing color, etc…
Bibliography
Lesson Design by Jordan School District Teachers and Staff.
Updated: 02/04/2018


 UTAH EDUCATION NETWORK
UTAH EDUCATION NETWORK

 Justin
Justin Braxton
Braxton Dani
Dani Kayla
Kayla Katie
Katie Matthew
Matthew Rob
Rob Val
Val
