Rate of Change in a Chemical Reaction
Summary
Students will establish a reaction rate for baking soda and vinegar and then change a variable to see the affect.
Materials
Attachments
- baking soda
- vinegar
- large test tube
- one hole stopper
- glass tube
- vinyl tubing
- 100 mL graduated cylinder
- safety glasses
- student worksheet
Instructional Procedures
Attachments
- The control can be established two ways. The students can perform the experiment and average their data to find the rate of the reaction. Or the teacher can demonstrate the experiment and establish a rate that way. It will take longer if the students perform the control experiment. The experiment is set up this way:
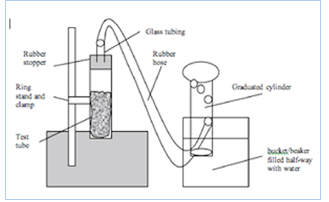
For the control, use 1 g baking soda, 10 mL vinegar, room temperature and 15 seconds of collection time. - List the variables on the board. The independent variables include the amount of baking soda and vinegar, the temperature of each, the temperature of the air (the reaction test tube could be placed in a cold or warm water bath) etc. Ask each student group to write a hypothesis that predicts how changing one of them would affect the dependent variable which is the volume of gas. For example: If we add 40 mL vinegar then the reaction will double in rate.
- Ask student groups to all write different hypothesis. As a class decide which are correctly written and make sense scientifically.
- Give students time to collect data. Each group should share with the class what they got for their dependent variable, the volume of the gas.
- Students can write up the experiment on their own or you can use the student sheet provided.
Bibliography
Lesson Design by Jordan School District Teachers and Staff.
Created: 10/10/2014
Updated: 02/01/2018
Updated: 02/01/2018
1502


 UTAH EDUCATION NETWORK
UTAH EDUCATION NETWORK

 Justin
Justin Braxton
Braxton Dani
Dani Kayla
Kayla Katie
Katie Matthew
Matthew Rob
Rob Val
Val
