A Water Cycle Chamber
Group Size
Small Groups
Summary
Classroom activity helps students understand the water cycle.
Materials
per group:
- clear 2-liter pop bottle with lid
- knife (teacher use)
- scissors
- ice cubes
- gooseneck lamp
- cup of warm water
- 1 plastic bag
Background for Teachers
Refer to Condensation Chambers (pdf) and Condensation Data Collection (pdf) in the Search for the Water Cycle Teacher's Edition.
Instructional Procedures
Attachments
Invitation to Learn
At the beginning of the day place a water bottle filled with ice and water on the table in the classroom. If students bring water bottles, each student could conduct this experiment. Throughout the day observe what happens to the outside of the water bottle. Discuss what causes the bottle to "sweat".
Instructional Procedures
- Demonstrate and review the water cycle using a commercial water cycle chamber of your own model.
- With each of the 2-liter pop bottles, use a knife to poke a starter hole in the top portion of the bottle where it starts to straighten out.
- Distribute one bottle to each group. Instruct the students to utilize the starter hole to cut off the top portion of the bottle with scissors. Tell them to look a the diagram below.
- Instruct each group to place a cup of very warm water in the bottom of the pop bottle. (Use hot water to speed the evaporation process.)
- Close the chamber with the top of the bottle inverted.
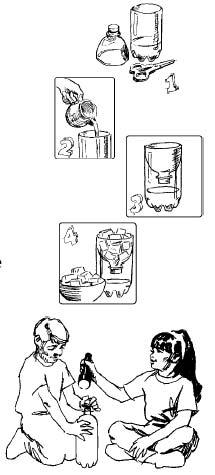
- Place four or five ice cubes in a plastic bag and set the ice cube bag on top of the container.
- Place the light source (lamp) within a few inches of the chamber to simulate heat energy from the sun. Be sure the area around the water is completely in the light.
- Notice how the top begins to fog. Remind students that the water vapor evaporating provides the moisture in the air allowing condensation to occur.
- After a couple of hours, the droplets on the ceiling of the bottle are so large that they begin to drip, simulating precipitation.
- Have the students record their findings.
Extensions
If resources permit, organize this activity as a take home science demonstration. Students can teach their family about the water cycle and report their experience to the class. You may choose to extend this activity by having the students mold landforms out of clay in the bottom their bottle. You may use the following recipe for Salt Ceramic Dough. Salt Ceramic Dough
Mix together:
- 1 cup of salt
- 1/2 cup corn starch
- 3/4 cup cold water
Stir constantly (about 2-3 minutes) until it becomes so thick that it follows the spoon in the stirring process, similar to bread dough. Spoon out the mixture onto foil or wax paper. When it is cool enough to handle, knead the dough for several minutes, and store in a sealed plastic bag until ready to use.
Assessment Plan
Check for students' understanding as they operate their chambers and do a class review of appropriate science language including: condensation, evaporation, precipitation, and water cycle. Engage students in a classroom discussion of the roles condensation, evaporation, and precipitation play in the water cycle.
Updated: 05/25/2022


 UTAH EDUCATION NETWORK
UTAH EDUCATION NETWORK

 Justin
Justin Braxton
Braxton Dani
Dani Kayla
Kayla Katie
Katie Matthew
Matthew Rob
Rob Val
Val
