Water Cycle
Summary
The teacher demonstrates how much usable fresh water there is available to plants and animals on earth and then demonstrates how the water cycle maintains itself with a water cycle demonstration. Students evaporate water with a hair dryer; observe condensation that forms on a mirror; observe a cloud in a bottle; and cause rain to occur in the lab.
Materials
Attachments
-
WaterCycle.doc
Student Worksheet -
WaterCycle.pdf
PDF version of student worksheet
- Water Cycle student sheet
- 5-gallon bucket
- Pyrex measuring cup
- eyedropper
- clear plastic container with a lid, about 3" x 6" (one for each group of students)
- cobalt paper, enasco.com SB16154 (A)M $15.95
- one small plastic cup that will fit inside the plastic container when the lid is closed
- squirt bottle with water
- wax paper
- hair dryer
- ice cubes
- two plastic Petri dishes or other small container
- thin mirrors
- 2L empty soda bottle with the label removed, one per group
- matches
- hotplate
- pot to boil water
- pie tin
Background for Teachers
Attachments
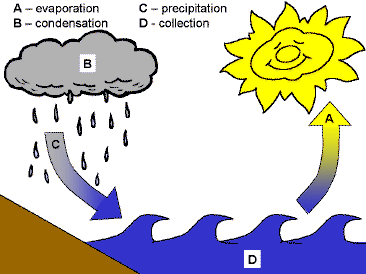
Nearly all the water on earth has been here since the earth was formed. It recycles through something called the water cycle. The water cycle is controlled by the Sun, which produces energy in the form of heat. This heat energy causes the water in the world's oceans, lakes, and even puddles in our backyard, to warm and evaporate. As the water evaporates into a vapor it rises in the atmosphere and cools. As the water cools high in the atmosphere it undergoes condensation back to a liquid and eventually forms a cloud. When the cloud gets heavy with water, precipitation occurs and water falls to the earth as rain. After the precipitation, the water evaporates and the process occurs all over again.
The amount of water vapor in the air varies with the time of day, weather, and location. Air with lots of water in it is said to be humid. Air without much water in it is said to be dry. How much water is in the air can be measured in a humidity chamber. Cobalt paper is an indicator for a humidity chamber and is blue when in dry air and turns pink in humid air.
Water changes its states based on the energy of its molecules. If their energy is low they will exist as solid ice, with more energy they exist as liquid water and the highest energy water molecules exist as a vapor or gas. In liquid water, when some of the liquid molecules get enough energy, they can escape the liquid phase and become a vapor even if the water isn't boiling. This is the process of evaporation. When vapor molecules of water cool their energy decreases and they change from the vapor state to the liquid state. This is called condensation. After precipitation, some of the water seeps into the soil and some of the water runs off over the land and into bodies of water such as rivers and lakes.
Intended Learning Outcomes
1c. Make simple predictions and inferences based upon observations.
1h. Use observations to construct a reasonable explanation.
3a. Know science information specified for their grade level.
3c. Explain science concepts and principles using their own words and
explanations.
4b. Report observation with pictures, sentences, and models.
4c. Use scientific language appropriate to grade level in oral and written
communication.
Instructional Procedures
Pre-lab discussion and demonstration: Look at an atlas and discuss how 70% of the earth is covered in water. Ask the students, if there is that much water on earth, will we ever not have enough water to drink? Perform the following demonstration for the students to illustrate that we have a lot of water on earth but there is very little of it available for us to drink. Discuss conservation of our water and reducing pollution so that we can keep our water drinkable.
- Take a five gallon bucket and fill it with water. This full bucket represents all the water on planet earth.
- Remove three cups of water (3%) in a 4 cup measuring cup. This represents all of the fresh water on the planet.
- Put two cups back in the five-gallon bucket (2%). This represents all of the fresh water frozen in the ice caps and glaciers, which we don't have access to.
- The one-cup that is left over represents the available fresh water on our planet. Much of this water is far below the surface of the earth.
- Squeeze five drops of this from an eyedropper (.8%). This is the amount of the fresh water available for our use. Show the students a poster of the water cycle and go through each of its steps. Discuss how the energy of water molecules is different in liquid and vapor states. This difference causes water to go through this cycle of evaporation, condensation, and precipitation.
Instructional Procedure:
I. Humidity Chamber
- Fill the plastic container with about 2 cm of warm water.
- Place a small piece of the cobalt paper in the small plastic cup. Be sure you do not get any water on the cobalt paper.
- Float the cup with cobalt paper in the dish with the warm water.
- Close the lid.
- Check the cobalt paper every five minutes as you do the other experiments and see if a color change occurs.
What is happening to the warm water in the container? The warm water has some water molecules moving so quickly that they leave the liquid state and become a vapor.
What is happening to the air inside the container? As the water evaporates it makes the air inside the container humid.
What caused the paper to turn pink? The water in the vapor causes the cobalt paper to turn from blue to pink.
What were we testing in this experiment? We were testing whether water was evaporating inside our chamber.
Take the pink paper out of the chamber and it will return to blue after it is left out for a few minutes. This paper can be reused.
II. Evaporation - The process in which water changes from a liquid to a vapor.
- Squeeze 5 small drops of water from the squirt bottle onto a piece of wax paper.
- Using a hair dryer on low setting, carefully blow on the drops of water.
What happened to your spots? They should disappear.
Where did the water go? The water changed from a liquid to a vapor. The water molecules are now floating around the room.
The hair dryer is acting as the warm sun and wind. This warmth gives more molecules the energy they need to leave the liquid state and move into the vapor or gas form. This is called evaporation.
III. Condensation - The process in which water changes from a vapor to a liquid.
- Place two ice cubes in a plastic Petri dish. The ice should be above the level of the plastic dish. Place the empty plastic Petri dish next to the one that contains the ice cube.
- Place the mirror, shiny side up, across the plastic dish containing the ice and a second dish that is empty. Notice that the under surface of the mirror rests on the ice cubes near one end.
- Let the mirror sit on the ice for 1-2 minutes.
What do you see forming on the top of the mirror above the ice? Students should see a fog of water droplets forming.
Where are these water droplets coming from? Did they come from the ice? Students may think the water droplets are moving from the ice, through the mirror to the other side. Be sure and explain that this is NOT happening. The water that is forming on top of the mirror is coming from the air around the mirror. As the air cools above the mirror, some of the water molecules in the air slow down enough that they leave the vapor form and become a liquid on top of the mirror. This process is called condensation.
What forms on the mirror as you breathe onto it? You should see a large amount of water droplets forming on the mirror because the water in your breath is undergoing condensation and forming water droplets on the mirror.
IV. Evaporation and condensation
- Open the 2L bottle and put in about 1 cup of lukewarm water. Be sure the water isn't too warm. Screw the cap on tightly. Swirl the water around the inside of the bottle so that most of the inside of the bottle is wet. Take the cap off the bottle.
- Have an adult light a match and blow it out. After it has gone out, immediately drop it into the water and screw the cap on tightly. Do not swirl the water in the bottle again.
- Squeeze the bottle for about 15 seconds as tightly as you can. Quickly let it go and look inside the bottle. See if you can observe a slight fog filling the bottle. You may need to do this several more times to be able to see the fog.
- Cycle back and forth between squeezing and letting go. Watch the cloud form, disappear, and reform.
Why is this happening? When you squeezed the bottle, you caused pressure and this pressure caused the liquid water to evaporate. When you released the pressure the water vapor went through condensation and formed as liquid droplets in the air in the center of the bottle. You change the water form a vapor to a liquid and back to a vapor each time you squeeze and release the bottle.
V. Precipitation -- When water that has undergone condensation in a cloud falls to the earth as rain or snow.
- Boil a pot of water on a hotplate. Be sure the students are aware of the hot
plate and stay away from it, they can be burned. Place ice into a dry Pyrex
measuring cup. Have an adult hold the Pyrex cup of ice over the pot of boiling
water.
What do you see forming on the bottom of the Pyrex cup? The students should see water droplets forming.
How did the water get on the bowl? The vapor coming off the boiling water hit the cold Pyrex cup and undergoes condensation.
- When you see water droplets forming on the outside of the pot with ice have someone carefully hold a pie tin or other container in between the ice and the boiling water. You should see it 'raining'.
What represents the 'clouds' in this experiment? The water droplets forming under the Pyrex dish represent a cloud.
What is precipitation? Rain
Why is this happening? Small misty drops condense on the underside of the bowl of ice. When the drops collide with each other they form bigger and bigger drops of water and represent clouds. When the clouds get enough water in them that they can no longer hold it, the water droplets drop down as rain onto the earth. If the temperature is cold enough, they drop as snow.
Bibliography
Rio Tinto Hands-on Science Curriculum Team
- Ms. Rae Louie -- Administrator, Principal Beacon Heights Elementary
- Emily Mortensen -- Grant writer, teacher outreach, 2nd grade teacher at Beacon Heights Elementary
- Ruth Li -- Curriculum design, K-6 Science Educator at Indian Hills Elementary
- Deirdre Straight -- Curriculum development, K-6 Science Educator at Beacon Heights Elementary
- Tim Rausch -- Website development, Library Media at Beacon Heights Elementary
Updated: 05/27/2022


 UTAH EDUCATION NETWORK
UTAH EDUCATION NETWORK

 Justin
Justin Braxton
Braxton Dani
Dani Kayla
Kayla Katie
Katie Matthew
Matthew Rob
Rob Val
Val
